Propecia (Finasteride) hair growth drug is used in the treatment of hair loss, popular worldwide. It is the Propecia brand name that makes Finasteride tablets to protect and regrow hair. Generally, common problems seem in both the gender (male/female), related to hair loss. Sometimes it happens due to dust & impure water, a few people have genetic issues. Also, you can buy Propecia finasteride hair growth online.
“Propecia is the treatment of male pattern hair loss, Only For men.”
According to studies, 85% of men are quite thin hair, and about 25% of men who suffer from male pattern baldness begin the painful process before reaching the age of 21 years.
The chemical from which Propecia made is very useful for the scalp and hair, now check out the look on the description.
Reason for Propecia finasteride hair growth popularity
Finasteride (active ingredient) present in Propecia tablets, which is a synthetic 4-asteroid compound. This compound is considered an inhibitor of steroid Type II 5α-reductase. 5α-reductase is an intracellular enzyme, which converts the androgen testosterone into 5α-dihydrotestosterone (DHT).
Empirical formula= C23H36N2O2
Molecular weight= 372.55
Melting point= 250°C
Propecia film-coated tablets containing 1 mg of Finasteride and lactose monohydrate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, hydroxypropyl methylcellulose, titanium dioxide, magnesium stearate & more.
Propecia finasteride hair growth drug reviews
-Mark (LA, Arrow Highway)
“ I’m a 35-year-old American male, I’ve had No side effects from this medicine, I’ve been using this drug for 3.5 years. In starting a few months, I don’t feel any changes in my hair from the last 3-4 months. But after 6 months, I’ve gained much new hair.”
-Charlie (Edinburgh, Albany Street)
“I am disappointed due to bald spots when I was 23 years. Tried rogaine but don’t get the benefit. At last, I choose Propecia, from a disappointed mind. Really, I feel amazed, within six months I started seeing results.”
-Oliver (Belfast, Abbeydale Crescent)
“My son takes this medicine since he was at 19 years. I am really thankful for this medicine, believe his hair has stopped thinning.”
Robert (Chicago, East ida B.Wells Drive)
“I just try this product after many experiments, for stopping my hair loss with some regrowth. Took nearly twenty years. detected some diminished sexual drive once a couple of years however not enough to be a tangle.”
Propecia finasteride hair growth drug cost & storage
Propecia is an inexpensive drug used in the treatment of hair loss and benign prostatic hyperplasia (BPH). The lowest price for Propecia is around $2.70, the average retail price of $65.60.
Detail of price and offer for 30 tablets of Finasteride 1 mg.
Provider of medicine have there a different scale of pricing, Checkout the list:
| Providers | Finasteride Price |
| Safeway | $79 |
| CVS Pharmacy | $77 |
| Target (CVS) | $77 |
| Rite Aid | $94 |
| Walgreens | $81 |
Variation in price 2019
| Date | Avg. Cash Price |
| May 2019 | $59.80 |
| Apr 2019 | $58.80 |
| Mar 2019 | $58.20 |
| Feb 2019 | $57.08 |
| Jan 2019 | $55.22 |
Storage:
- The drug should be kept at room temperature between 59°F to 86°F (15°C to 30°C)
- Moisture free (Keep Propecia drug Dry)
- Try to keep the drug in a closed container
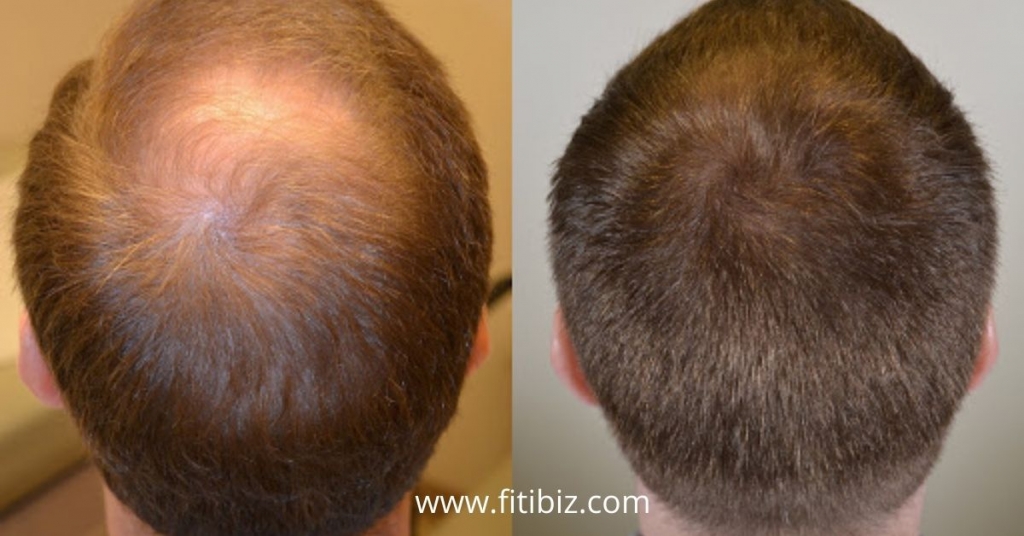
Propecia finasteride hair growth Drug Result
Propecia uses for the treatment of hair loss, now you are going to know about the tips which will help to get quick hair growth. Only made for males and approved by the FDA, in trend from 1997 as a treatment for hair loss.
80% of the men who used Finasteride in the clinical trials didn’t experience any more hair loss, 66% of them regrow hair over an extended period.
Finasteride doesn’t work overnight, takes time, and requires consistent usage for the best results of Propecia. In the process of regrowing hair, we can’t see the major results until they’ve taken Finasteride for at least a year or more.
1. Massage your scalp daily
According to Japanese researchers, a daily four- minutes massage over the hair increases blood circulation and helps to stimulate hair growth and thickness.
2. Take biotin supplements
Vitamin B complex, ensuring that your hair, fingernails, and skin stay healthy. Lacking biotin hair of growing weaker and more slowly than usual
3. Mix Finasteride with minoxidil for better result
Along with Finasteride, it’s the only other product approved by the FDA. According to observation on 984 men, various stages of a male pattern have observed 5% minoxidil to their balding spots twice a day.
Side effects of Propecia finasteride hair growth
A coin has a two-face. And same has with Propecia medicine. Like any other medicine, this medicine also has some side effects, and below we have listed some of the side effects of the medicine:
- Decrease blood Prostate Specific Antigen (PSA), an increase in the following PSA levels from their lowest point may indicate the presence of prostate cancer and it should be evaluated
- Weakness/Helplessness
- Loss of interest in sex
- Orgasm problem
- Swelling in body
- Weakness/dizziness
- Headache
- Runny nose
Quick doctor consults are suggested because if it is a symptom of male breast cancer. If you are suffering from any of the below-given issues, consult your doctor as soon as it is possible:
- Breast lumps
- Tenderness with breast pain
- Nipple discharge
- Changes in male breast size
Common Side Effects Of Propecia finasteride hair growth
- Abnormal Premature Ejaculation Causes (abnormal ejaculation),
- The problem of having an orgasm (the climax of sexual excitement),
- Not a feeling of any excitements,
- Impotence,
Propecia finasteride hair growth drug dosage & Precautions
- Propecia drug can be taken with or without meals (empty stomach).
- Daily use of this drug for three months or more is necessary, to check out the results.
- Don’t recommend this pill to anyone without the advice of a doctor.
- Don’t take this drug more than recommended.
Precautions
- Before taking medicine, tell your doctor about any other allergies, you are suffering from.
- Check your heart and blood health before taking drugs.
- Protect from child/don’t throw medicine anywhere in a public place or room.
- Avoid giving this medicine during pregnancy.
Complications And Warnings of Propecia finasteride hair growth drug
Should Not Take By Women
Propecia drug is not meant for treatment in women. Therefore women should not touch broken or crushed tablets of Propecia. Most apparently when a woman is pregnant or may possibly be pregnant. Because the chance of absorption of finasteride (Propecia) may cause the subsequent possible danger to a male fetus.
However, Propecia medicines are well coated. Therefore it can prevent your contact with the active ingredient while normal handling of this drug. Besides that, the tablets must have not been crushed or broken. Although you should aware of use In specific populations, indications, usage, contradictions, and how supplied to patient information.
Impacts On PSA (Prostate-Specific Antigen)
Although in some clinical studies of Propecia known as finasteride 1 mg, in the 18-41 years of the age group of men. In that study, the mean value of serum prostate-specific antigen (PSA) is reduced from 0.7 ng/mL at baseline to 0.5 ng/ml in a 12 months period of time.
Moreover, in clinical studies with Proscar known as finasteride, 5 mg. When this drug is used in older men who have benign prostatic hyperplasia (BPH). Then the PSA levels may be decreased by approximately 50%. However, some other studies with Proscar revealed that it can also be caused some reductions in serum PSA in the appearance of prostate cancer.
Although these conclusions must be taken into account for proper analysis of serum PSA when estimating those men who treated with finasteride. Therefore any confirmed progress from the lowest PSA value while on Propecia may signal the presence of prostate cancer.
In addition, it must be evaluated, even if PSA levels are still inside the normal range for those men who are not taking a 5α-reductase inhibitor. Besides that, the non-compliance to treatment with Propecia can also affect the PSA test results.
Chance Of High-Grade Prostate Cancer
Although it can increase the chance of high-grade prostate cancer with 5α-reductase Inhibitors. During the study in men, the aged group of 55 and over with a regular digital rectal examination and PSA ≤3.0 ng/mL at baseline using finasteride 5 mg/day. That is 5 times the dose of Propecia.
This dose in the 7-year Prostate Cancer Prevention Trial known as PCPT had an enhanced chance of Gleason, that score 8-10 prostate cancer. However, similar results were inspected in a clinical trial of 4-year placebo-controlled with another 5α-reductase inhibitor known as dutasteride.
However, the 5α- reductase inhibitors can increase the chance of improvement of high-grade prostate cancer. Whether the impact of 5α-reductase inhibitors to decrease the volume of the prostate or study-related circumstances, affected the outcomes of these studies, which has not been established.
Higher Risk For Male Fetus
Physicians must need to inform patients that those women who are currently pregnant or can be pregnant in the coming future must not handle split or fragmented Propecia tablets. Because of the feasibility of incorporation of finasteride into the skin and the subsequent potential risk to the fetus of a male.
Although such tablets of Propecia drugs are normally coated. So that it can also prevent such contact with the vital ingredient of this drug during ordinary handling. But it provided that the pills have not been crushed or broken.
Besides that, if a pregnant woman or a potentially pregnant woman get in touch or contact with any of the broken Propecia tablets. Then, the touched or contact part or skin of the body should be immediately washed with water and soap
Increased Risk Of High-Grade Prostate Cancer: Patients must be notified that there was an increment in high-grade prostate cancer in such men treated with 5α-reductase inhibitors that were indicated for BPH treatment when compared to those patients treated with placebo in studies looking at the use of these drugs to prevent prostate cancer.
Some More Instructions
Physicians must instruct all of their patients to promptly report any differences in their breasts such as pain, lumps, or nipple discharge. Al these breast changes also including tenderness, breast enlargement, and neoplasm have been reported.
Some other important effects of this drug or the usages are given below:
Nursing Mothers: Profecia drug is not meant for usage in women. It is not known whether finasteride drug is excreted in human milk or not.
Pediatric Use: Profecia medication is not designated for application in pediatric patients. Although the safety and effectiveness of this drug in pediatric patients have not been confirmed.
Geriatric Use: However, some clinical effectiveness studies with Profecia did not involve subjects aged between 65 and above. Although based on the pharmacokinetics of finasteride 5 mg drug, no adjustment of dosage is required for the elderly for Profecia. However, the effectiveness of Profecia in the elderly has not been established.
Hepatic Impairment: Although caution must be exercised in the administration of Profecia in such patients who come with some liver function irregularities, as finasteride drug is metabolized largely in the liver.
Renal Impairment: However, no adjustment of the dosage is required for those patients who are with some renal impairment.




Write a comment
Your email address will not be published. All fields are required